

The revised USP <797>, which became official on November 1, 2023, clarifies the provisions for immediate-use compounding, including changes in the number of components used, the beyond-use date (BUD), and expectations for training and competency.
USP <797>, from the United States Pharmacopeia, is a set of enforceable sterile compounding standards. It applies to compounding pharmacies and facilities involved in the preparation of compounded sterile preparations (CSPs). The intent of USP 797 is to prevent patient harm and death through minimum practice and quality standards expected of any entity or individual involved in storage, handling, preparation and transportation of CSPs. (Compounded Sterile Preparations).
USP <800> is established in concert with USP General Chapters <795> for non-sterile compounding and USP <797> for sterile compounding. The purpose of the chapter is to provide standards and define processes to protect personnel, patients, and the environment when handling hazardous drugs in a healthcare setting.
HPI's certified technicians are prepared to conduct all testing in accordance with guidelines to assist facilities in maintaining their compliance.
Call us for all:
Ante Room
Buffer Rooms
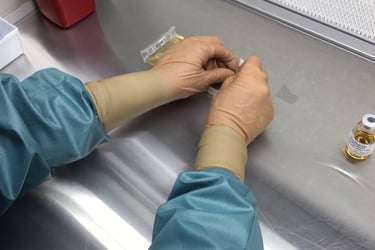
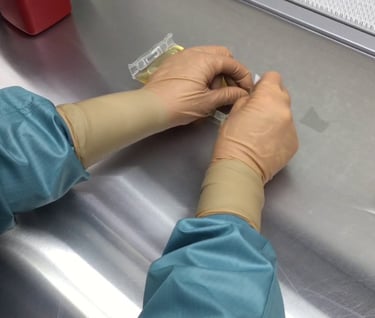
Personnel testing (Garbing & GFT {gloved fingertip})
Media Fill testing
LAFWs
BSCs
HEPA leak testing
Viable and Non-Viable air sampling
Differential pressure testing
Installations/Decommissioning of equipment
Hazardous drug contact sampling
TAB {Testing and Balancing}
and more..
As a certified VOSB, we are certified to work in all VA's and other government facilities.



Call or text 813-291-3344
8051 N. Tamiami Trail
STE E6
Sarasota, FL. 34243




or - click or scan our QR code:




